SULFETOS
Fonte: http://geology.csupomona.edu/drjessey/class/gsc215/minnotes17.htm
The sulfides form an important class of minerals that includes the majority of the ore minerals. With them are classed the similar but rarer sulfarsenides, arsenides, and tellurides. Most sulfide minerals are opaque with distinctive colors and characteristically colored streaks. Those that are nonopaque, such as cinnabar, sphalerite and orpiment, have extremely high refractive indices and transmit light only on thin edges.
The general formula for the sulfides is given as XmYn in which X represents the metallic elements and Y the nonmetallic element. The sulfides can be divided into small groups of similar structures but it is difficult to make broad generalizations about their structure. Regular octahedral or tetrahedral coordination about sulfur is found in many simple sulfides such as in galena, PbS, (with an Nalco-type structure), and in sphalerite, ZnS. In more complex sulfides, as well as sulfosalts, distorted coordination polyhedra may be found. Many of the sulfides have ionic and/or covalent bonding, whereas others, displaying most of the properties of metals, have metallic bonding characteristics. The important sulfides are listed in the Table below:
|
SULFIDES, SULFARSENIDES, AND ARSENIDES |
|||
| Acanthite | Ag2S | Cinnabar | HgS |
| Chalcocite | Cu2S | Covellite | CuS |
| Molybdenite | MoS2 | Sphalerite | ZnS |
| Galena | PbS | Stibnite | Sb2S3 |
| Pyrite | FeS2 | Marcasite | FeS2 |
| Chalcopyrite | CuFeS2 | Bornite | Cu5FeS4 |
| Pyrrhotite | Fe1-xS | Pentlandite | (Fe,Ni)9S8 |
| Arsenopyrite | FeAsS | ||
Acanthite/Argentite - Ag2S polymorphs, argentite the high T monoclinic form and acanthite the low T isometric form. Acanthite is the chief ore of silver, but nonetheless is not a common sulfide mineral.

Occurrence - Associated with mesothermal vein mineralization of complex mineralogy including chalcopyrite, bornite, pyrite, sphalerite and galena. The veins often exceed 10 feet in width and are comprised largely of quartz, calcite, dolomite and other carbonates with lesser sulfide. Most famous localities of are Mexican silver districts of Guanajuato and San Luis Potosi. It is estimated that 75% of the world's silver production has come from central Mexico. In the U.S., Tonopah and the Comstock Lode produced appreciable silver from acanthite ores. More recently acanthite was been recovered as a byproduct from the copper ores of Butte, Montana and the Coeur d'Alene.
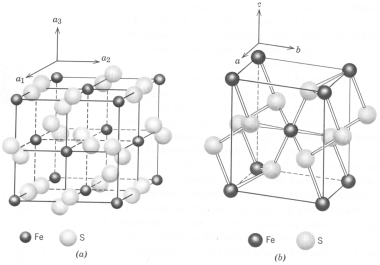
Sphalerite - ZnS occurs in two polymorphic forms: the sphalerite-type and the wurtzite-type structures. In both the sphalerite and wurtzite structures zinc is surrounded by four sulfurs in tetrahedral coordination, but in sphalerite the zinc atoms are arranged in a face-centered cubic lattice (see below), whereas in wurtzite they are approximately in positions of hexagonal closest packing. The atomic arrangement in sphalerite is like that in diamond in which the carbon has been replaced by equal amounts of Zn and S. Natural sphalerite shows extensive Fe2+ and very limited Cd2+ substitution for Zn. Sphalerite crystals are tetrahedral, but are generally malformed and often etched. Resinous luster is present in some sphalerites, but often absent in iron-rich wurtzite.
Occurrence - Sphalerite is the chief ore of zinc. It has two major modes of occurrence:

- Mississippi Valley deposits - occurs in association with galena in a series of carbonate-hosted ore deposits throughout the Mississippi Valley and eastern U.S. The east and central Tennessee districts have been major producers in the U.S., as has the Tri-State district of Oklahoma-Kansas-Missouri.
- Massive Sulfide deposits - these deposits are far less common in the U.S., but account for most of the world's zinc production. Sullivan, British Columbia, Kidd Creek, Ontario and Mt. Isa/Broken Hill in Australia are the largest of these mines. The ores are a mixture of fine-grained sulfides in volcanic or sedimentary rocks. They are thought to be analogous to present day black smokers at divergent plate margins.
Uses - Zinc is utilized for galvanizing iron, as an alloy with copper in brass and in pharmaceutical products, particularly vitamins.
Galena - Galena is the chief ore of lead. It has the NaCl structure and as such forms cubes and octahedrons. Galena often occurs as well-formed crystals and much more rarely as massive granular aggregates.

Occurrence - Galena is a ubiquitous mineral in most sulfide ore deposits. It has been mined from several different types of deposits, paradoxically all are younger the 1 billion years of age.

- Mesothermal Vein deposits - Galena is a common constituent
of mesothermal veins, but it has been mined from these
deposits not for the lead, but for silver in solid solution
within the galena. Famous districts are Coeur d'Alene, Idaho,
Silverton and Cripple Creek, Colorado.
- Mississippi Valley deposits - Most of the world's lead
production comes from mines in the Viburnum Trend of southeast
Missouri.
- Massive Sulfide deposits - Although massive sulfide
deposits are known for their zinc and copper production, a few
such as Kuroko, Japan have been major lead producers.
Uses - Utilized for the manufacture of lead-acid batteries, lead shot and piping, and reactor shielding. Recognized to be toxic to humans if ingested, many of its previous uses are now banned including lead crystal, lead-based paint and as an additive to gasoline.
Chalcopyrite - CuFeS2, has a structure that can be derived from the sphalerite structure by regularly substituting Cu and Fe ions for Zn; this leads to a doubling of the unit cell. Stannite, Cu2FeSnS4, has a structure based on sphalerite in which layers of ordered Fe and Sn alternate with layers of Cu (chalcopyrite can be rewritten as Cu2Fe2S4; this illustrates chemically the close similarity to Cu2FeSnS4). Chalcopyrite occurs as small tetrahedral crystals, often with striated faces.

Occurrence - Chalcopyrite and bornite (See below) are the chief ores of copper and occur in a variety of settings:
- Porphyry Copper deposits - About 50% of the world's copper
comes from porphyry copper deposits, most in North and South
America. About 75 deposits occur in a broad band stretching
from southern Arizona into Utah. The chalcopyrite was
deposited from hydrothermal solutions associated with felsic,
porphyritic intrusives of Mesozoic age. Pyrite and molybdenite
and commonly associated minerals.
- Massive Sulfide deposits - Many massive sulfide deposits
in Canada and southern Europe have produced appreciable copper,
the most famous on the Isle of Cyprus.
- Mesothermal Vein deposits - Although known largely for
their silver production, some mesothermal vein systems, such
as Butte, Montana have produced mostly copper.
- Mississippi Valley deposits - These deposits produce
mostly galena and sphalerite, but some have recovered
appreciable byproduct copper.
Bornite - Cu5FeS4 is rarely crystalline occurring as massive, granular aggregates. It is often easily recognizable since it tarnishes to a "purple-blue hue" giving the mineral its common name of "Peacock Ore". Bornite is much less common than chalcopyrite. Its occurrences are as above for chalcopyrite.
Covellite - CuS, (hexagonal) is an example of a chemically very simple substance with a rather complex structure in which part of the Cu is tetrahedrally coordinated by four S, and part coordinated by three S in the form of a triangle. The structure can, therefore, be viewed as made of sheets of CuS3 triangles, between double layers of CuS4 tetrahedra; covalent sulfur-sulfur bonds link the layers. The net result is hexagonal prisms


Occurrence - Both covellite and chalcocite (See below) are important ores of copper. Both are largely supergene in origin. They form when primary (hypogene) copper deposits of chalcopyrite and bornite are weathered. The copper goes into solution and is carried downward by ground water until it encounters reducing conditions. Reduction of the copper results in deposition of covellite. Many hypogene copper deposits would be uneconomic were it not for the chalcocite/covellite supergene enrichment blanket over the primary ore.
Chalcocite - similar to covellite, but can be deposited as a primary sulfide mineral. Also occurs as hexagonal platy crystals.


Cinnabar - HgS (hexagonal) is the chief ore of mercury. It is also highly sought after by mineral collectors since specimens are often attractive and good crystals are quite rare, cinnabar usually occurring as drusy coatings on other minerals.

Occurrence - Cinnabar forms in hot springs environments and since it is unstable (vaporizes) even at low temperature does not persist in rocks older than Tertiary age. It is closely associated with chalcedony, amethyst, pyrite and calcite. The most famous locality is Almeden in Spain were cinnabar has been mined continuously since Roman times. The district is still active although more money is made from the sale of pyrite specimens these days. Currently most cinnabar is mined in China. During the early 20th century San Benito County, CA was the largest mercury producer in the world, but environmental problems closed all of the mines.
Uses - Mercury is used in electrical switches and the amalgamation of silver and gold in the third world. Because of extreme toxicity mercury mining is banned in the U.S.
Pentandite - (Fe,Ni)9S8 is the chief ore of nickel. It forms complex isometric face centered unit cells that can have variable Fe-Ni ratios as well as appreciable cobalt. It is virtually unknown as well formed crystals.

Occurrence - Pentlandite is commonly associated with pyrrhotite and chalcopyrite in ultramafic rocks. The world's largest deposit of nickel occurs at Sudbury, Ontario. The deposit is unique in that it formed by meteor impact. It is estimated that 50% of the world's nickel is concentrated in this deposit. Nickel has also been recovered from Archean-age komatiites, ultramafic extrusives, in Australia and Canada. The famous of the komatiite mines are near Kambalda, West Australia.
Uses - The chief use of nickel is in the manufacture of steel. Small amounts of nickel are added to increase the toughness and strength. Nickel is also alloyed with other metals to produce coinage and for plating.
Stibnite - Sb2S3 often occurs as spectacular radiating clusters of prismatic crystals. It is the chief ore of antimony.

Occurrence - Like cinnabar, stibnite is found in hot springs deposits, but it is much rarer. Most of the world's antimony is mined in China, but Japan produced many of the spectacular crystal specimens on display in museums early in the 20th century. Minor antimony has also been mined in the Andes Mountains. Most antimony actually is recovered for the smelting of lead ore so little stibnite is mined these days.
Uses - Antimony is used to make specialty-tinted glass.
Molybdenite - MoS2 usually occurs in thin sheets and as smears along fracture. Good crystals are rare. It is often confused with graphite due to similar hardness, color and its ability to write on paper

Occurrence - The United States has a virtual lock on the world's reserve of molybdenum, estimated at 85%. Most occurs in a band of a dozen deposits from central Colorado to northern New Mexico. Of these Climax, Colorado is the best known. Another belt of deposits occurs in southern Alaska. The moly is present in porphyry moly deposits that are in most respects similar to porphyry copper deposits, indeed copper is sometimes recovered as a byproduct. Many moly deposits also have appreciable cassiterite and wolframite from which tin and tungsten are recovered.
Uses - Molybdenum is used in electrical circuits and as an alloy with many other metals.
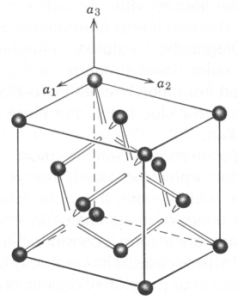
Pyrite and Marcasite - These minerals are polymorphs of FeS2; the former isometric and the latter orthorhombic. The figure below shows the structure of pyrite (a) and marcasite (b). The polymorphic relationship of the minerals has defied all study. Most think marcasite is the low temp polymorph, but pyrite forms readily in reducing environments at the Earth's surface. Furthermore, much marcasite is highly unstable when exposed to the atmosphere and decrepitates quickly. Pyrite favors well-formed crystals, often cubic, but just as commonly pyritohedrons. Marcasite also forms crystals, but is more common as cockscomb intergrowths of botryoidal aggregates.
Occurrence - pyrite is a very common mineral occurring as:
- common constituent of sedimentary rocks formed in reducing environments
- a common mineral in low to moderate temperature hydrothermal deposits. Spectacular specimens like the one below from Spain are common.
- accessory mineral in plutonic rocks formed from O-poor magmas

Marcasite has a more restricted mode of occurrence. Despite its synthesis at low temperature in the lab, natural marcasite seems to occur in only low temperature hydrothermal ore deposits.
Uses - None, in fact a major environmental hazard. Pyrite and marcasite oxidize easily at the Earth's surface producing strongly acidic solutions (acid mine drainage). These solutions have often had a devastating effect on the environment.
Pyrrhotite - Fe1-xS (monoclinic) is essentially the high temperature disordered equivalent of pyrite. Its disorder results from missing sulfur in some lattice sites. Until recently, pyrrhotite was not thought to occur in well-formed crystals. During the past few years well-formed prismatic crystals have been coming out of Russia. Because of their rarity the best specimens have commanded a premium. Pyrrhotite is commonly associated with pentlandite and lesser chalcopyrite. It constituents the chief gangue mineral in all high temperature nickel deposits. Like pyrite it has no commercial value, but fortunately it does not oxidize readily and produces little environmental damage.

Arsenopyrite - FeAsS (monoclinic) A common gangue mineral in many high temperature gold deposits including the famous Homestake mine at Lead, South Dakota. Rarely forms large crystals, favoring granular aggregates. Arsenopyrite has been recovered during smelting since it would be hazardous to release arsenic into the environment. The arsenic is used in medicines, insecticides and glass coloring agents. Because it contains arsenic, arsenopyrite is one of those minerals that have received a bad rap. Unlike orpiment and realgar (the other common arsenic-bearing minerals), arsenopyrite is remarkably stable and has never been shown to cause significant pollution or toxicity problems.
Fonte: http://geology.csupomona.edu/drjessey/class/gsc215/minnotes17.htm
 |
imprimir |



